Cell Size & Developmental Decision Making
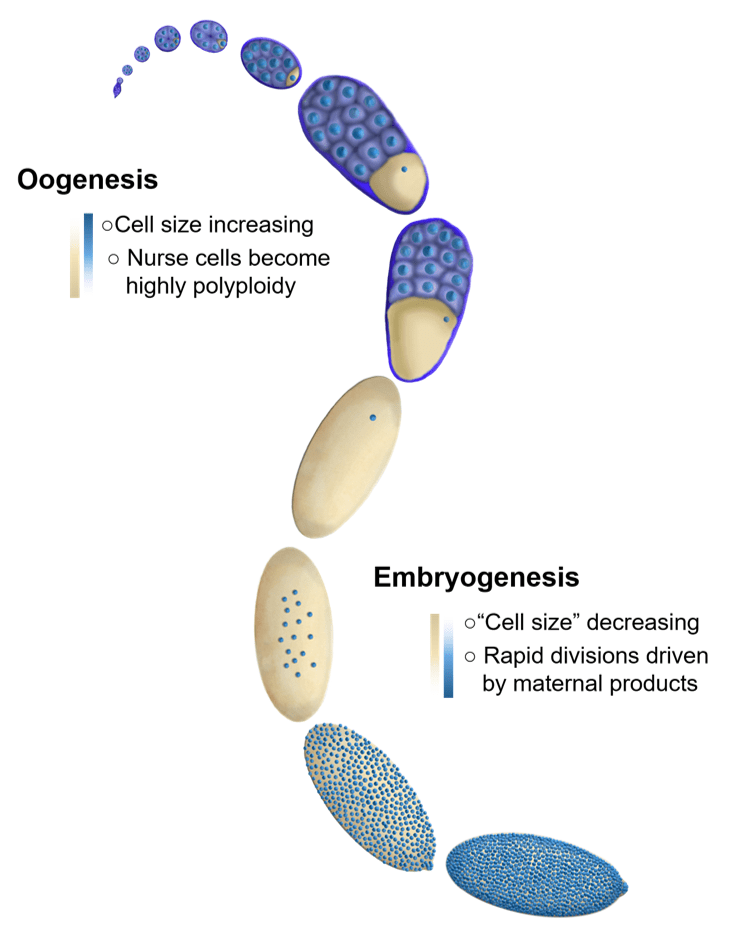
We are broadly interested in how cells coordinate growth, division and differentiation. Much of the research in our lab focuses on the oocytes and early embryos of the fruit fly, Drosophila. The embryos of species (such as flies) with very large eggs face a very large challenge. They need to immediately divide their cytoplasm into a more manageable volume. In order to do this, embryos undertake several rapid rounds of cleavage divisions before beginning to grow and differentiate. Interestingly, in many species these extremely rapid divisions are almost entirely driven by maternally loaded proteins and RNAs as the embryo is globally transcriptionally repressed until the cell cycles lengthen. Similarly, the early cleavage cycles lack cell cycle checkpoints and the cells remain non-motile. The transition from the rapid cleavage phase, to the beginning of differentiation is known as the mid-blastula transition, or MBT. In Drosophila, this transition is accompanied by cellularization since the early cleavage divisions are syncytial.
Coordination between the number of cell divisions and the events of the MBT is critical for proper development. We are interested in understanding the general biological principals that underlie the sensing of cell size, regulation of developmental progression, and control of transcription during this dynamic time period in development. Our research broadly falls into four categories outlined below:
1. Cell size and the Cell Cycle
Fundamentally, cell size control requires the coupling of cell volume to cell division. In a typical, growing cell this means that the cell must double in volume before initiating division. However, this typical paradigm is inverted in the early embryos of many species. The newly fertilized embryo is single cell with a single diploid nucleus that, in the case of externally developing organisms like flies, fish, and frogs, must contain all of the material (RNA, protein, lipids, metabolites etc.) to produce whatever is going to hatch out of that egg. Therefore, the newly fertilized egg is an enormous single cell. The first order of business for these giant cells is to divide their cytoplasm into more manageable cell sizes through a series of reductive cleavage divisions. When the cells reach the correct size they switch gears from dividing to differentiating. In flies this switch is incredibly robust and happens at the same time for the entire embryo (during the 14th cleavage division). We seek to understand how the cells know when to stop dividing and start differentiating.
Our work indicates that one way that the cells measure cell size/developmental progression is through the changing ratio of maternally loaded histone protein to nuclei. Most proteins, including histones are provisioned in large amounts by the mother during oogenesis to support the rapid early cleavage divisions. In the case of histones, depletion of the maternally provided pool is directly coupled to cell number (which is inversely proportional to cell size) though histone chromatin incorporation. When these histone pools are exhausted, the cell cycle slows and transcription initiates. A recent breakthrough in our lab indicates that the remaining pool size of maternally provided histones may be sensed through direct inhibition of a cell cycle regulatory kinases by the histone H3 tail. This inhibition is independent of chromatin incorporation. This surprising observation opens many novel areas of inquiry into how histones may act as regulatory molecules off of chromatin. Current projects in the lab explore the biochemical details of how histones directly interact with cell cycle regulators as well as other potential roles for histones as signaling molecules off of chromatin.
2. Chromatin
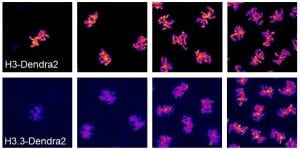
The chromatin environment of the early embryo is highly dynamic. Initially the DNA is constantly either in a state of replication or mitotic condensation. The chromatin in these cells is largely undifferentiated (analogous to the state of embryonic stem cells in mammals), with very little transcription, no heterochromatin, and no topologically associated domains (TADs). This all changes in the cycles leading up to the MBT in which transcription initiates, heterochromatin is specified, and TADs form for the first time. Recently, we have found that the replication-coupled H3 is titrated against the increasing number of genomes and replaced by replication-independent H3.3. Moreover we can alter the number of divisions before zygotic genome activation by altering the maternal loading of replication-dependent histones. Ongoing projects in the lab seek to understand how chromatin composition changes during development, differentiation, and aging and to elucidate the functional consequences of these changes.
3. Zygotic Genome Activation
Transcription initiation is not an instantaneous event. Rather, it occurs over a period of several cell cycles and is spatially regulated for many genes. Moreover, different genes become activated at different times and with different kinetics. We are interested in understanding how different genes know when and where to be activated and what genomic features underlie different activation behaviors. Ongoing projects in the lab seek to understand how different perturbations affect the initial activation of the whole genome using sequencing technologies and individual genes at very high temporal resolution through imaging.
4. Control of size during oogenesis
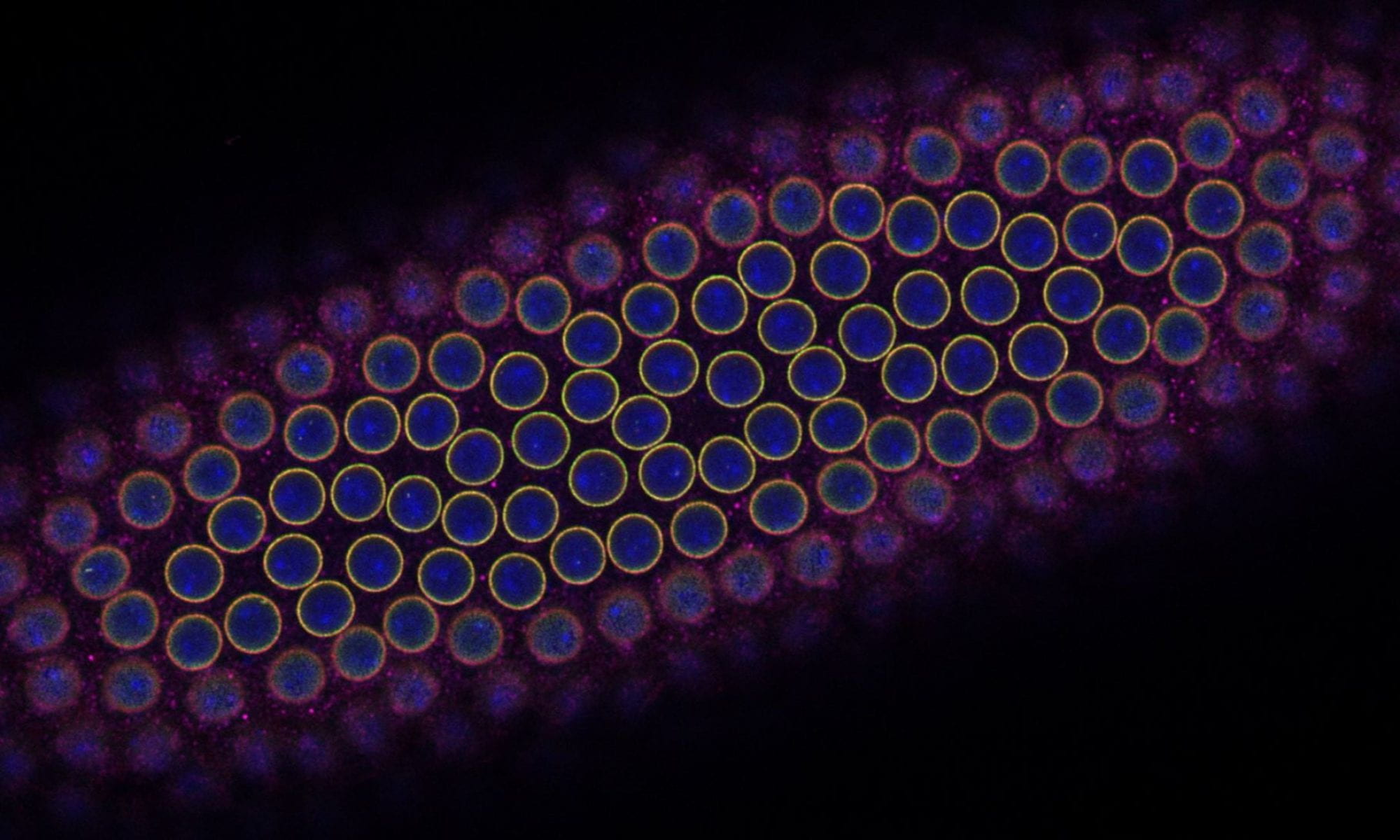
The oocyte has the reverse problem of the egg. It needs to provide all of the materials required to produce the massive egg cell. It does this by utilizing 15 “helper” cells called nurse cells that become hugely polyploid to allow for the necessary biosynthetic capacity to make the egg. These 16 cells (15 nurse cells and 1 oocyte) together with an enveloping epitaxial layer form a simple organ called the egg chamber. Once the nurse cells have grown large enough, they push their cytoplasm into the growing oocyte in a process called nurse cell dumping. If the amount of material provided by the nurse cells is insufficient, the resulting egg will be too small to be viable. How do the nurse cells know that they’ve reached the correct size to produce a viable egg? How do 16 individual cells coordinate their behaviors to measure their total size? Ongoing projects focus on exploring how organ size is sensed in the growing egg chamber as well as how size signals are transmitted between nurse cells.