Much of the work in my lab focuses on the study of surface-attached microbial communities known as biofilms. These surface attached communities can be found in medical, industrial and natural settings. In fact, life in a biofilm represents the predominate mode of growth for microbes in most environments. Biofilm microbes are typically surrounded by an extracellular matrix, which provides structure and protection to the community. Biofilm-grown bacteria are notorious for their tolerance to a range of antimicrobial agents including clinically relevant antibiotics. Key goals of my lab are to understand how biofilms form in the context of both living (i.e., the cystic fibrosis airway) and non-living (i.e., medical implant) surfaces. We are also interested in the role of biofilms in host-pathogen interactions and tolerance to antibiotic therapy. In recent years, the lab has started to explore polymicrobial interactions in the context of airway and intestinal communities in cystic fibrosis.

A central interest in the lab is the understanding the role of surface sensing as bacteria transition from a planktonic (free-swimming) mode of growth to life in a biofilm. Using the model organism Pseudomonas aeruginosa, an opportunistic human pathogen that causes a variety of diseases in immune compromised patients, we have asked the question, "Can bacteria sense a surface?" We think the answer is yes! Our work has shown that "surface sensing" requires the bacterial pilus and two key second messengers: cAMP and c-di-GMP. For these studies, we work with a biophysicist, Gerard Wong at UCLA to combine molecular genetics, single cell tracking and theoretical frameworks to understand how microbes engage with and attach to surfaces (check out this and this as examples of our collaborative studies). We have shown recently that the Type 4 pilus associated protein PilY1 is likely the first documented mechanosensor in bacteria required for surface sensing. We recently reviewed mechanosensing and biofilm formation in bacteria. We continue to explore the mechanisms of surface sensing with our trans-disciplinary team.
The microbiome and polymicrobial interactions: in the lab and in the clinic. In recent years we have explored the polymicrobial communities associated with airway infections common to the disease cystic fibrosis (CF). We have sought to develop new, more complex polymicrobial models of infection. A recent workshop report has helped define these new directions in the lab, and we have proposed approaches to tackling this challenging problem here and here. We recently described our first efforts at leveraging large clinical data sets to develop and use a new, and we thinking really exciting, in vitro polymicrobial model of CF.
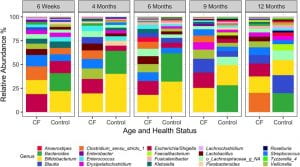 The gut microbiome in infants and children with CF. I also work closely with Juliette Madan and Julie Sanville, physician-scientists at Dartmouth, who are tracking a cohort of infants and children with CF from birth to >8 years of age. This work has uncovered a striking and surprising finding — the best predictor of airway disease outcome is the microbial communities found in the gut. We recently reviewed the literature support this so-called "gut-lung axis". A report from our team showed that one of the microbes depleted in infants and children with CF is the important immune modulating genus Bacteroides. Our ongoing microbiome studies are geared towards a better understanding of the link between gut and airway communities, including working with mouse models with our collaborators in the Cramer, Bliska and Ross labs here at Dartmouth.
The gut microbiome in infants and children with CF. I also work closely with Juliette Madan and Julie Sanville, physician-scientists at Dartmouth, who are tracking a cohort of infants and children with CF from birth to >8 years of age. This work has uncovered a striking and surprising finding — the best predictor of airway disease outcome is the microbial communities found in the gut. We recently reviewed the literature support this so-called "gut-lung axis". A report from our team showed that one of the microbes depleted in infants and children with CF is the important immune modulating genus Bacteroides. Our ongoing microbiome studies are geared towards a better understanding of the link between gut and airway communities, including working with mouse models with our collaborators in the Cramer, Bliska and Ross labs here at Dartmouth.

How do microbes regulate biofilm formation via the c-di-GMP network and a large cell surface adhesin? The second messenger cyclic-di-GMP regulates biofilm formation by the soil microbe Pseudomonas fluorescens. This soil microbe uses cyclic-di-GMP to regulate the localization of a large surface adhesion called LapA, which is critical for biofilm formation. Our work has uncovered a novel "inside-out" signal transduction pathway that allows the cell to control localization of this adhesin in response to changing pools of cyclic-di-GMP inside the cell. We work closely with Holger Sondermann, a structural biologist and biochemist at Cornell, to understand the structural basis of this signaling pathway. We have also employed atomic force microscopy to understand how the large adhesin LapA allows this microbe to initiate biofilm formation. Recent studies have focused on which parts of LapA contribute to attachment, and how this protein is anchored to the cell surface.
We also are exploring the c-di-GMP regulatory network in this organism, as there are 50+ proteins that make, break or bind this second messenger. A key goal in the field is to understand how the cell can coordinate the action of all of these players. Towards this end, we reported a network analysis that combined assaying 50+ mutants for biofilm formation under ~180 growth conditions, testing the expression profiles of these genes in ~50 growth conditions, and performing 2000+ bacterial two hybrid assays. This study suggested that the cell uses a combination of regulatory mechanisms to control the network output. We have recently teamed with Peter Mucha's group in the Math Department at Dartmouth to better model the in's and out's of this signaling network.