Molecular and mechanical mechanisms of epithelial folding
Apical constriction is a commonly used mechanism to achieve epithelial folding during tissue morphogenesis. During apical constriction, constricting cell apices shrink and the basal ends of the constricting cells expand, resulting in a characteristic wedge-like shape. Such cell shape changes are accompanied by folding of the epithelial sheet into a 3-dimensional tissue. Apical constriction mediated epithelial folding is a well conserved process and occurs in a variety of developmental contexts. While we have a detailed understanding of how constriction forces are generated near the apical surface of cells, it remains elusive how cells respond to apical constriction to change shape in 3D, and how an “in-plane” constriction drives “out-of-the-plane” bending of the tissue.
Mechanisms of 3D cell shape changes induced by apical constriction
We use Drosophila mesoderm invagination as a model to study epithelial folding. During Drosophila gastrulation, ventrally localized mesoderm precursor cells become internalized by forming a ventral furrow. The cells first constrict their apices and elongate in the apical-basal direction (“the lengthening phase”). During their subsequent invagination, they shorten back to a wedge-like morphology (“the shortening phase”). Previous work reveals that during apical constriction, the tissue interior behaves as a viscous continuum and flows as the tissue constricts apically, mediating the apical-basal lengthening of the ventral cells (He et al., 2014). Interestingly, during this process, the lateral cell membranes promptly expand with the flow without obstructing the flow, suggesting that the viscous deformation of the tissue interior is enabled by an active mechanism that promotes cell membrane expansion, as a response to apical constriction. These findings led us to investigate how cells perceive and respond to mechanical forces to facilitate coherent tissue deformation. In particular, since cell membrane adjustment usually requires active regulation of intracellular trafficking, we seek to understand how mechanical stimuli influence intracellular trafficking and how such regulation impacts tissue mechanics.

Mechanisms of “out-of-the-plane” tissue bending
Our recent work demonstrates that during mesoderm invagination, actomyosin contractility is critical to prevent tissue relaxation during the early, “priming” stage of folding but is dispensable for the actual folding step after the tissue passes through a stereotyped transitional configuration. This binary response suggests that Drosophila mesoderm is mechanically bistable during gastrulation, analogous to an elastic sheet under compression. Based on results from computer modeling and experimental measurements, we propose that Drosophila mesoderm invagination is achieved through an interplay between local apical constriction and mechanical bistability of the epithelium that facilitates epithelial buckling (Guo et al., 2022). In line with this model, we find evidence suggesting a role for ectodermal compression in Drosophila mesoderm invagination. Furthermore, we find that the effective transition from apical constriction to invagination requires tissue-level mechanical coordination between the constricting and non-constricting domains. Depletion of the apical-basal polarity determinant Dlg1 impairs the mechanical integrity of cells adjacent to the constricting domain and weakens the tissue-level mechanical coordination, causing a delay in invagination (Fuentes and He, 2022). These findings lead to several important follow-up questions. How is tissue compression generated in the ectodermal tissue during gastrulation? How does Dlg1 regulate subcellular organizations that enable tissue level mechanical coordination? And finally, how do developmental programs regulate tissue properties both in space and in time?

Feedback between apical actomyosin and intracellular trafficking
Our recent work demonstrates an actomyosin-dependent mechanism involving Rab11-mediated trafficking in regulating apical constriction in the Drosophila embryo. During Drosophila mesoderm invagination, apical actin and Myosin II (actomyosin) contractility induces apical accumulation of Rab11-marked vesicle-like structures (“Rab11 vesicles”) by promoting a directional bias in dynein mediated vesicle transport. At the apical domain, Rab11 vesicles are enriched near the adherens junctions (AJs). The apical accumulation of Rab11 vesicles is essential to prevent fragmented apical AJs, breaks in the supracellular actomyosin network and a reduction in the apical constriction rate. Importantly, This Rab11 function is separate from its role in promoting apical Myosin II accumulation. These results suggest an actomyosin-dependent, Rab11-mediated feedback mechanism that serves to reinforce the connections between the actomyosin network and apical AJs. This mechanism allows the tissue to promptly adapt to the increased tissue tension during apical constriction and maintain the integrity of the force generation machinery in the process of tissue folding (Chen and He, 2022). Moving forward, we seek to understand how apical actomyosin induces biased transport of Rab11 vesicles and how these vesicles regulate AJs.

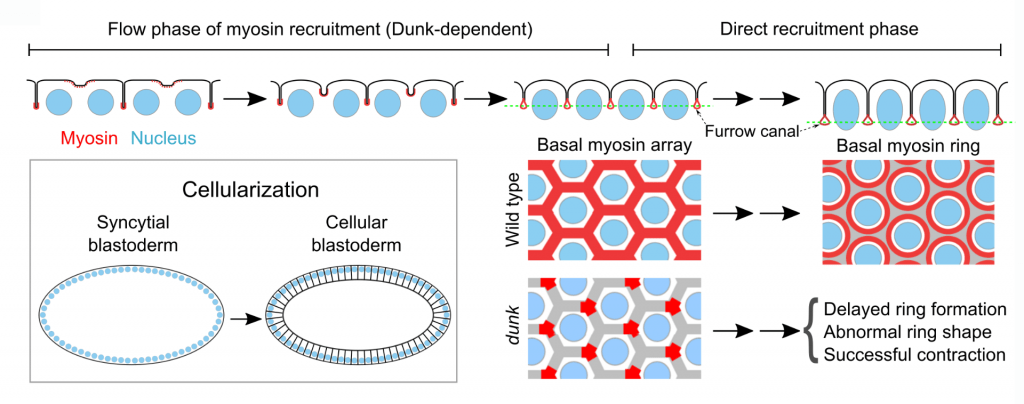
Zygotic regulation of cortical myosin during cellularization
Drosophila cellularization is a special form of cleavage that converts syncytial embryos into cellular blastoderms by partitioning the peripherally localized nuclei into individual cells. Similar to canonical animal cytokinesis, an early event in cellularization is the recruitment of non-muscle myosin II (“myosin”) to the basal tip of cleavage furrows, where myosin forms an interconnected basal array before reorganizing into individual cytokinetic rings. The initial recruitment and organization of basal myosin are regulated by a cellularization-specific gene, dunk,but the underlying mechanism is unclear. Through a genome-wide yeast two-hybrid screen, we identified anillin (Scraps in Drosophila), a conserved scaffolding protein in cytokinesis, as the primary binding partner of Dunk. We further found evidence suggesting that Dunk regulates myosin recruitment and organization during early cellularization by interacting with and regulating anillin. We seek to understand how Dunk coordinates with other anillin regulators, such as RhoA and PI(4,5)P2, to regulate the function of anillin, as well as how anillin’s function in myosin recruitment and organization is differentially regulated in cellularization (interphase of the cell cycle) and in typical animal cytokinesis (anaphase-telophase).