Recombinant production of TEV cleaved human parathyroid hormone.
Abstract
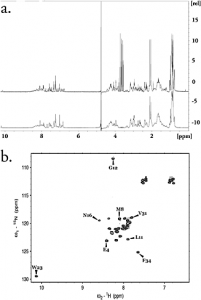
The parathyroid hormone, PTH, is responsible for calcium and phosphate ion homeostasis in the body. The first 34 amino acids of the peptide maintain the biological activity of the hormone and is currently marketed for calcium imbalance disorders. Although several methods for the production of recombinant PTH(1-34) have been reported, most involve the use of cleavage conditions that result in a modified peptide or unfavorable side products. Herein, we detail the recombinant production of (15) N-enriched human parathyroid hormone, (15) N PTH(1-34), generated via a plasmid vector that gives reasonable yield, low-cost protease cleavage (leaving the native N-terminal serine in its amino form), and purification by affinity and size exclusion chromatography. We characterize the product by multidimensional, heteronuclear NMR, circular dichroism, and LC/MS.