IG-NIRS provides deep tissue functional characterization at high resolution. This approach combines conventional imaging techniques such as MRI and CT with optical NIR technologies, giving information directly relating to the vascular and metabolic status of tissue in-vivo.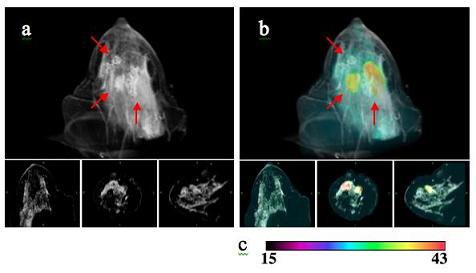
Image-guided near infrared spectroscopy (IG-NIRS) provides deep tissue functional characterization at high resolution. This approach combines conventional imaging techniques such as MRI and CT with optical near infrared technologies, giving information directly relating to the vascular and metabolic status of tissue in-vivo. The resultant estimates of total hemoglobin, oxygen saturation, water, lipids and scatter provide a window towards understanding the mechanisms of cancer in terms of angiogenesis, hypoxia, changes in the interstitium and cell organelle structural changes. This type of spectroscopy has been applied for breast cancer diagnosis and treatment monitoring, as well as image-guided fluorescence in small-animals.
Optimization of these systems is essential to provide quantitative and accurate spectroscopy. This optimization encompasses system design for simultaneous multi-modality image acquisition, methods for intelligently combining spatial anatomical structure from MRI/CT into optical recovery, image segmentation, visualization and interpretation of novel combined optical and MRI/CT parameters.
The focus of this project is optimization to go from image segmentation of MRI volume to recovery of spectroscopic parameters in 3-D in a seamless automated manner, in 3-D. Funded by NIHNIBIB, computationally efficient models have been developed using finite element (FE) and boundary element methods (BEM). BEM is specific to IG-NIRS using only surface discretization whereas FEM allows for NIR tomography using volume discretization. Both models solve for light propagation in tissue using diffusion equation. Software packages have been developed using FEM (NIRFAST) and BEM (BEMFAST) to run in multi-processing cluster. Segmentation tools and visualization based on ITK and VTK have been developed specific to this multi-modality setting.
Our goals are to study breast tissue in-vivo and monitoring different tissue responses to neoadjuvant chemotherapy using this multi-modality approach. Cancer shows higher total hemoglobin compared to benign lesions. In addition, total hemoglobin levels appear to be sensitive to how the patient is responding to neoadjuvant chemotherapy. We are also studying image-guided fluorescence in small animals as a precursor to clinical work in fluorescence.