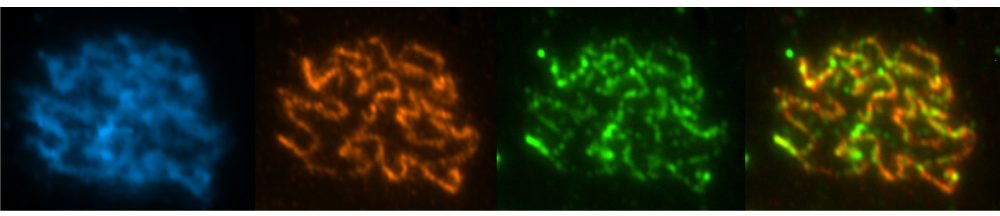
One of the most critical aspects of cell division is the accurate partitioning of genetic material into the daughter cells. During DNA replication, connections between the newly formed sister chromatids are established. This “cohesion” between sisters is required for proper orientation of chromosomes on the mitotic spindle and when cohesion is released during anaphase, the sisters are able to segregate to opposite poles. Regulation of cohesion (establishment, maintenance and release) is a critical part of every cell cycle.
During meiosis, cohesion not only holds sisters together, but also plays an essential role in meiotic recombination and maintaining the association of recombinant homologues until anaphase I. The release of cohesion during meiosis is also unique—arm cohesion is released during anaphase I but centromeric cohesion must be maintained until anaphase II. Therefore, regulation of meiotic cohesion requires additional control mechanisms that are not present during mitosis.
 Defects in meiotic cohesion lead to the production of aneuploid gametes. In humans, chromosome segregation errors during female meiosis are the leading cause of birth defects and miscarriages. In addition, the incidence of meiotic segregation defects increases dramatically as women age. By the time a woman reaches her early forties, she has a 1 in 3 chance of conceiving an aneuploid fetus. Although the correlation between increased maternal age and segregation errors is well-established, the underlying molecular defects that cause reduced fidelity of chromosome segregation in older oocytes are largely unknown. Because human oocytes undergo meiotic recombination during fetal development and remain suspended in a prolonged prophase I arrest until ovulation (which can be 10-50 years later), the continuous association of homologous chromosomes and their accurate segregation during anaphase I demands that meiotic sister-chromatid cohesion remain intact for decades. Therefore, deterioration of meiotic cohesion with age may be one prominent cause of age-dependent nondisjunction (NDJ) in human oocytes. Work in our lab supports this hypothesis.
Defects in meiotic cohesion lead to the production of aneuploid gametes. In humans, chromosome segregation errors during female meiosis are the leading cause of birth defects and miscarriages. In addition, the incidence of meiotic segregation defects increases dramatically as women age. By the time a woman reaches her early forties, she has a 1 in 3 chance of conceiving an aneuploid fetus. Although the correlation between increased maternal age and segregation errors is well-established, the underlying molecular defects that cause reduced fidelity of chromosome segregation in older oocytes are largely unknown. Because human oocytes undergo meiotic recombination during fetal development and remain suspended in a prolonged prophase I arrest until ovulation (which can be 10-50 years later), the continuous association of homologous chromosomes and their accurate segregation during anaphase I demands that meiotic sister-chromatid cohesion remain intact for decades. Therefore, deterioration of meiotic cohesion with age may be one prominent cause of age-dependent nondisjunction (NDJ) in human oocytes. Work in our lab supports this hypothesis.
 Our long-term goal is to define the pathway of events necessary for the proper regulation of sister-chromatid cohesion and chromosome segregation during meiosis and to understand the molecular events that cause reduced fidelity of meiotic chromosome segregation in older oocytes. We are using the model system Drosophila melanogaster to understand the mechanisms that govern meiotic cohesion and chromosome segregation. Analysis of meiosis in fruit flies allows us to capitalize upon a number of genetic techniques to identify proteins required for normal segregation and cytological methods to monitor the morphology and behavior of meiotic chromosomes. Because the process of meiosis is so highly conserved, our research provides a basic framework to understand the defects in meiotic chromosome segregation that lead to disease in humans.
Our long-term goal is to define the pathway of events necessary for the proper regulation of sister-chromatid cohesion and chromosome segregation during meiosis and to understand the molecular events that cause reduced fidelity of meiotic chromosome segregation in older oocytes. We are using the model system Drosophila melanogaster to understand the mechanisms that govern meiotic cohesion and chromosome segregation. Analysis of meiosis in fruit flies allows us to capitalize upon a number of genetic techniques to identify proteins required for normal segregation and cytological methods to monitor the morphology and behavior of meiotic chromosomes. Because the process of meiosis is so highly conserved, our research provides a basic framework to understand the defects in meiotic chromosome segregation that lead to disease in humans.