Understanding Early-life Influenza Antibody Repertoire Imprinting Through Infection or Vaccination
PI: Jiwon Lee, PhD
Ralph and Marjorie Crump Assistant Professor of Engineering
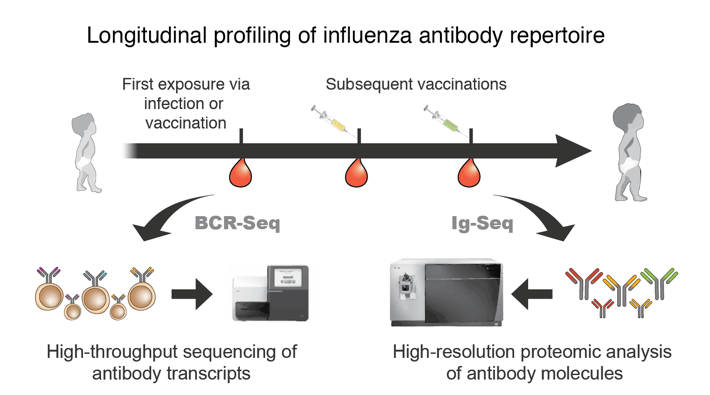
Influenza is a rapidly evolving virus that poses persistent global health threat. Due to the ubiquitous nature of influenza, almost all children are exposed to influenza virus by the age of 6. From this early exposure to influenza via infection or vaccination, the first influenza antibody repertoire is established, and it is reshaped continuously through repeated exposures to a multitude of different influenza virus strains during one’s lifetime. Recent studies based on epidemiology, modeling, and traditional serology metrics have suggested that the initial antibody repertoire generated from the first exposure is ‘imprinted’ in the immune system. The constraints imposed by the imprinted antibody repertoire exert a major influence on the nature of the antibody response elicited upon subsequent challenges, potentially impeding the effectiveness of novel vaccines. For this project, we will track the longitudinal continuity of anti-influenza antibody repertoires as a function of initial exposure route and test the hypothesis that the first exposure through infection results in stronger imprinting of initial influenza antibody repertoire than the first exposure through vaccination.
Understanding the Role of Meiotic Misregulation in Germ Cell Tumor Formation
PI: Soni Lacefield, PhD
Professor of Biochemistry and Cell Biology
During female reproduction, eggs are made through the process of oogenesis, which couples cellular differentiation with the specialized cell division process of meiosis. During meiosis, chromosome replication is followed by two meiotic divisions in which chromosomes segregate in each division. During meiosis I half the chromosomes are extruded into a polar body and half remain in the egg. In meiosis II, the egg arrests just prior to segregation to await fertilization. Only upon fertilization is meiosis II completed, extruding half the chromosomes into another polar body. How eggs remain in a meiosis II arrest is poorly understood, but previous studies have shown that a failure to maintain the arrest causes poor fertility and germ cell tumors. This project studies mouse oogenesis to understand the mechanisms of meiosis II regulation to determine how the egg stays in a state that is competent for fertilization. In addition, the project will determine how germ cell tumors form when eggs fail to maintain the arrest.

Understanding the role of RNA-binding protein mutations in cancer
PI: Prerna Malaney, PhD
Assistant Professor of Biochemistry and Cell Biology
The discovery of therapeutically actionable genetic lesions in malignancies has revolutionized patient care. However, many cancers lack targetable driver mutations or experience relapse after targeted therapies, leading to limited treatment options and a poor overall prognosis. We suggest that identifying non-traditional drivers of malignant transformation can unveil new therapeutic targets. To identify potential drivers, we relied on a recently published survey of the COSMIC cancer mutation database and found that approximately 5% of all mutated genes (n=65) in cancer are RNA-binding proteins (RBPs). RBPs interact with multiple target RNAs and regulate post-transcriptional processing, including splicing, poly-adenylation, degradation, and translation. The overarching goal of my research is to elucidate the role of RBPs in key cellular processes, establish the role of RBP mutations in tumorigenesis, and pinpoint therapeutic vulnerabilities in downstream signaling pathways. The culmination of these studies will strengthen the understanding of the role of RBPs in crucial biological processes and the involvement of dysregulating mutations as drivers of disease.
