Small Molecule Inhibition of the Na+/H+ Exchange Regulatory Factor 1 and Parathyroid Hormone 1 Receptor Interaction.
Abstract
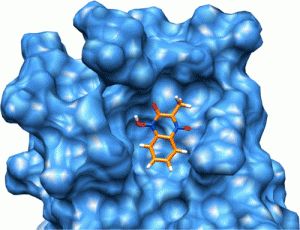
We have identified a series of small molecules that bind to the canonical peptide binding groove of the PDZ1 domain of NHERF1 and effectively compete with the association of the C-terminus of the parathyroid hormone 1 receptor (PTH1R). Employing nuclear magnetic resonance and molecular modeling, we characterize the mode of binding that involves the GYGF loop important for the association of the C-terminus of PTH1R. We demonstrate that the common core of the small molecules binds to the PDZ1 domain of NHERF1 and displaces a 15N-labeled peptide corresponding to the C-terminus of PTH1R. The small size (molecular weight of 192) of this core scaffold makes it an excellent candidate for further elaboration in the development of an inhibitor for this important protein-protein interaction.