J Biol Chem. 2015 290(5):2879-87. doi: 10.1074/jbc.M114.609768. PMID: 25492869
"Phosphorylation of Ezrin-Radixin-Moesin-binding Phosphoprotein 50 (EBP50) by Akt Promotes Stability and Mitogenic Function of S-phase Kinase-associated Protein-2 (Skp2)."
Song GJ, Leslie KL, Barrick S, Mamonova T, Fitzpatrick JM, Drombosky KW, Peyser N, Wang B, Pellegrini M, Bauer PM, Friedman PA, Mierke DF, Bisello A.
Abstract
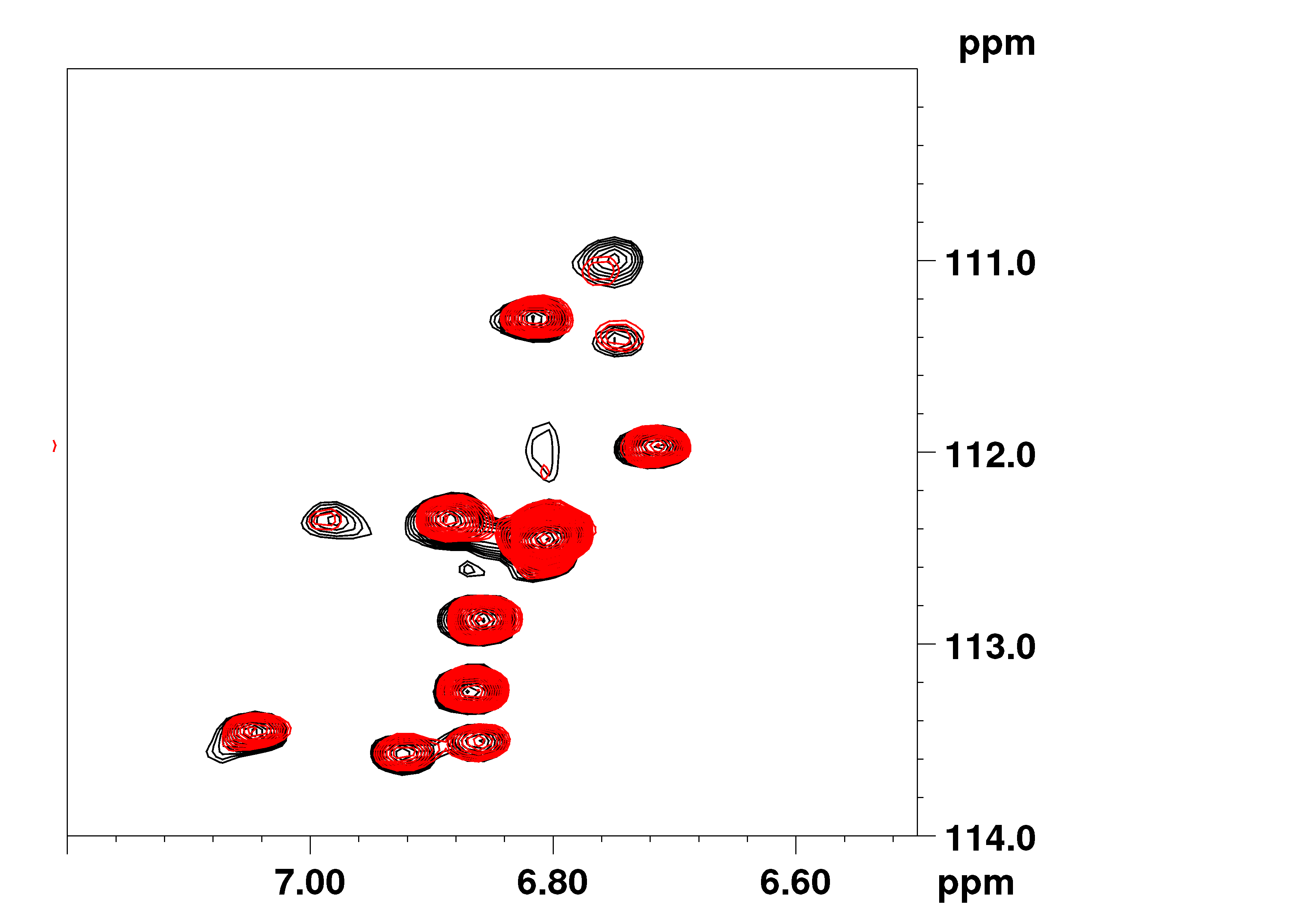
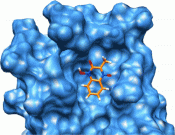
The regulation of the cell cycle by the ubiquitin-proteasome system is dependent on the activity of E3 ligases. Skp2 (S-phase kinase associated protein-2) is the substrate recognition subunit of the E3 ligase that ubiquitylates the cell cycle inhibitors p21(cip1) and p27(kip1) thus promoting cell cycle progression. Increased expression of Skp2 is frequently observed in diseases characterized by excessive cell proliferation, such as cancer and neointima hyperplasia. The stability and cellular localization of Skp2 are regulated by Akt, but the molecular mechanisms underlying these effects remain only partly understood. The scaffolding protein Ezrin-Binding Phosphoprotein of 50 kDa (EBP50) contains two PDZ domains and plays a critical role in the development of neointimal hyperplasia. Here we report that EBP50 directly binds Skp2 via its first PDZ domain. Moreover, EBP50 is phosphorylated by Akt on Thr-156 within the second PDZ domain, an event that allosterically promotes binding to Skp2. The interaction with EBP50 causes cytoplasmic localization of Skp2, increases Skp2 stability and promotes proliferation of primary vascular smooth muscle cells. Collectively, these studies define a novel regulatory mechanism contributing to aberrant cell growth and highlight the importance of scaffolding function of EBP50 in Akt-dependent cell proliferation.