Protein Engineering of the N-terminus of NEMO: structure stabilization and rescue of IKKβ binding.
Abstract
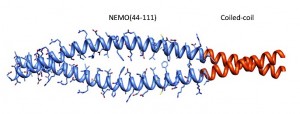
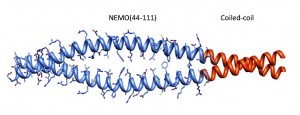
NEMO is a scaffolding protein that, together with the catalytic subunits IKKα and IKKβ, plays an essential role in the formation of the IKK complex and in the activation of the canonical NF-ĸB pathway. Rational drug design targeting the IKK binding site on NEMO would benefit from structural insight, but to date the structure determination of unliganded NEMO has been hindered by protein size and conformational heterogeneity. Here we show how the utilization of a homodimeric coiled-coil adaptor sequence stabilizes the minimal IKK binding domain NEMO(44-111) and furthers our understanding of the structural requirements for IKK binding. The engineered constructs incorporating the coiled-coil at the N-terminus, C-terminus or both ends of NEMO(44-111) present high thermal stability and cooperative melting, and most importantly restore IKKß binding affinity. We examined the consequences on structural content and stability by circular dichoism and nuclear magnetic resonance and measured binding affinity of each construct for IKKβ(701-745) in a fluorescence anisotropy binding assay, allowing us to correlate structural characteristics and stability to binding affinity. Our results provide a method to engineer short stable NEMO constructs to be suitable for structural characterization by NMR or X-ray crystallography. Meanwhile the rescuing of the binding affinity implies that a pre-ordered IKK-binding region of NEMO is compatible with IKK binding and the conformational heterogeneity observed in NEMO(44-111) may be an artifact of the truncation.
http://www.ncbi.nlm.nih.gov/pubmed/25286246