Small-molecule inhibitors of JC polyomavirus infection.
Abstract
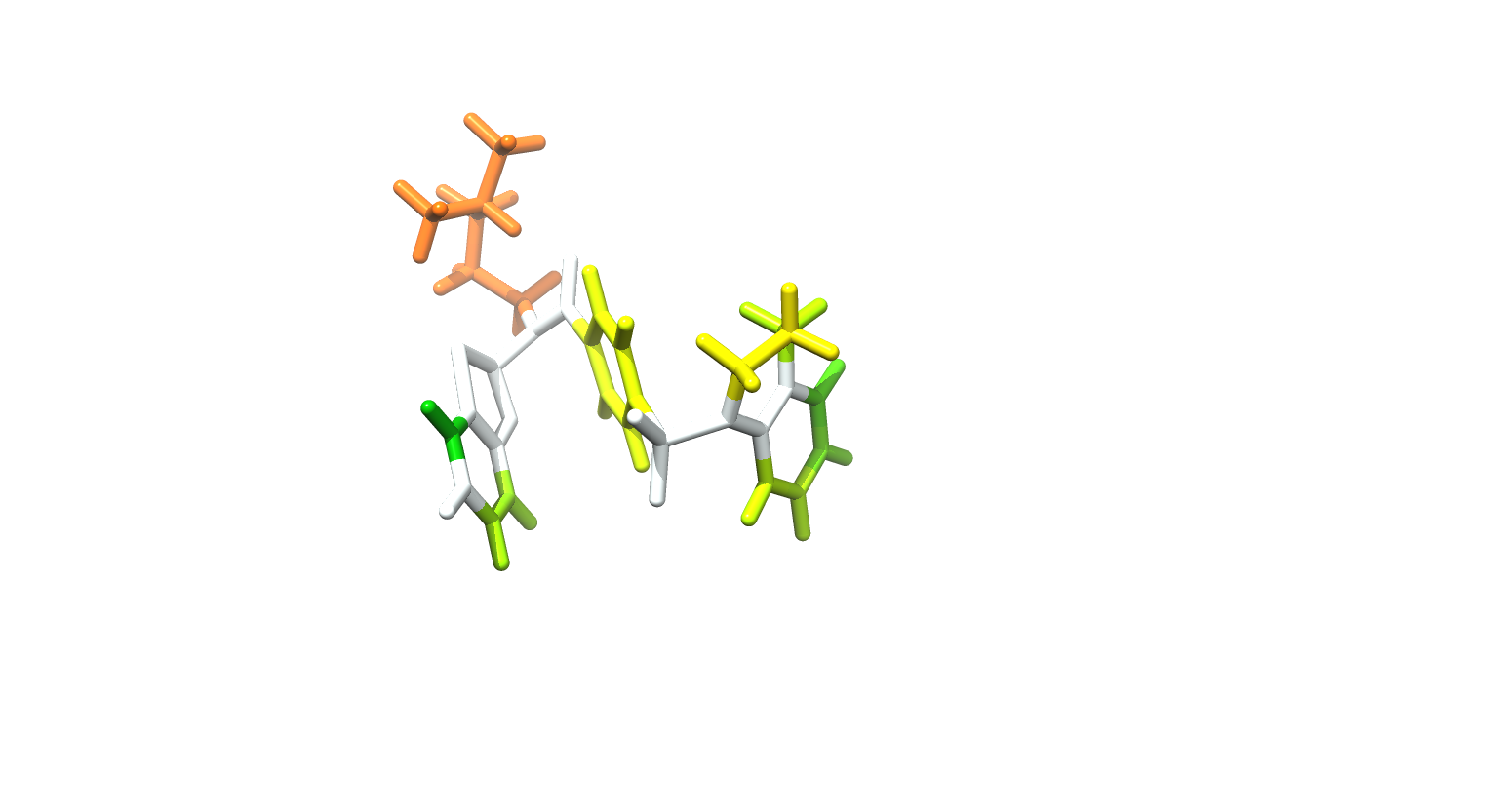
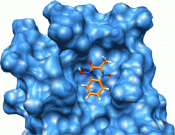
The JC polyomavirus (JCPyV) infects approximately 50% of the human population. In healthy individuals, the infection remains dormant and asymptomatic, but in immuno-suppressed patients, it can cause progressive multifocal leukoencephalopathy (PML), a potentially fatal demyelinating disease. Currently, there are no drugs against JCPyV infection nor for the treatment of PML. Here, we report the development of small-molecule inhibitors of JCPyV that target the initial interaction between the virus and host cell and thereby block viral entry. Utilizing a combination of computational and NMR-based screening techniques, we target the LSTc tetrasaccharide binding site within the VP1 pentameric coat protein of JCPyV. Four of the compounds from the screen effectively block viral infection in our in vitro assays using SVG-A cells. For the most potent compound, we used saturation transfer difference NMR to determine the mode of binding to purified pentamers of JCPyV VP1. Collectively, these results demonstrate the viability of this class of compounds for eventual development of JCPyV-antiviral therapeutics.
http://www.ncbi.nlm.nih.gov/pubmed/25522925