Urtica dioica contains medically useful antiviral and anti-inflammatory agents, and thus understanding factors that influence production of such compounds may prove beneficial. In Cerro de la Muerte, Costa Rica, it is found in both shade and light conditions. I tested the hypothesis that it adjusts allocation between leaf growth and defense according to resource availability. Leaf size, stinging hair properties, and herbivory test differed with light conditions. Results indicated further patterns in anti-herbivore defenses related to leaf maturation.
Introduction
Herbivory pressure has influenced plants to evolve with structural, chemical, and other forms of defenses. Among these, much attention has been given to secondary metabolites that potentially have pharmaceutical or industrial uses. Urticaceae is a stinging nettle family known for its stinging trichomes that incorporate both physical and chemical defenses. It is found in high elevations such as around the biological station of Cerro de la Muerte, Costa Rica, and one species (Urtica dioica) is locally used as an anti-inflammatory aid for arthritis and rheumatism. A number of studies have reported its antiviral activity against HIV, diuretic and hypotensive effects, and medicinal usefulness for treating prostatitis and prostate hyperplasia (1-3). The species appears to have adapted to both light and shade environments, and understanding the nature of chemical defenses along different quality habitats may help further current understanding of how biotic and abiotic factors control the quality and quantity of plants’ defense strategies.
One hypothesis is that the limited availability of light in shade may make the production of leaves more costly and cause the plants to invest more in defense quality or quantity to reduce their losses. Alternatively, the plants exposed to more sunlight may make more anti-herbivore investment due to the abundant resources, while the shade plants focus more on leaf growth and sacrifice anti-herbivory characteristics. I predicted that to compensate for the limited light, the shade plants would invest more in leaf growth, and thus have larger leaf size, show lower number of stinging hairs, and suffer greater herbivory, while the light plants would exhibit opposite trends.
Methods
Feeding Trial and Selection of the Plant and Herbivores
To determine which insects consume medicinal plants, I subjected Orthopterans, Coleopterans, and larval Lepidopteras to a 12-hour long feeding trial, using leaves from Winteraceae, Verbenaceae, and Urticaceae. Only plant species that showed signs of herbivory and the insects that consumed them were used for the feeding test. The selected herbivores were Orthoptera: Tettigoniidae (grasshoppers) and larval Lepidoptera (caterpillars).
Herbivory Test
A total of 26 grasshoppers and 14 caterpillars were starved for 9 hours, and then placed individually into a 14 x 17 cm Ziploc bag blown with air, with a small wet cotton ball. Urticaceae leaves were sampled from 12 plants in sunny, open environment and 12 plants in shade environment. The species was identified as U. dioica (4, 5). Young leaves were defined as the first sprouts on the 1st or 2nd node exceeding 2.5 cm in length, and old leaves as the first sprouts on the 4th or older node. The sizes of the old leaves in both conditions were controlled, and the sizes of the young leaves in both conditions were controlled separately. I excluded leaves previously fed upon by folivores. Four leaves were evenly distributed inside each bag: light and young, light and old, shade and young, and shade and old. Each trial lasted for 24 hours, and 12 additional hours if there was no sign of herbivory. The conditions of all subjects were observed up to 36 hours after the feeding trial to discover any visibly malignant consequences for consuming the leaves.
Leaf Comparison
I sampled 121 undamaged leaves from 12 plants in sunny environments and 12 plants in shady environments. Young leaves were defined as the first sprouts on the 1st node, adolescent leaves as the first sprout on the 2nd node, and old leaves as the first sprouts on the 4th-5th nodes. Length and width were measured for each leaf, and I counted the number of stinging hairs on one half of the lower surface of each leaf.
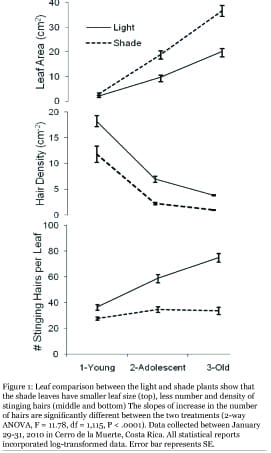 Results
Results
Herbivory Test
Thirteen grasshoppers and 10 caterpillars did some feeding during the trial (58% of the total sample). For all 23, the favored leaves were in the order of light-old, shade-old, and young leaves in light or shade environment (x2 = 14.04, df = 1, P < .001). They also preferred the light leaves over shade leaves (binomial probability for 15 of 23 = 0.06), and the old leaves over young leaves (binomial probability for 19 of 23 < 0.001). Grasshoppers and caterpillars exhibited different preferences; grasshoppers followed the overall pattern of preference for the light and old leaves (binomial probabilities for 9 of 13 = 0.09 and for 10 of 13 = 0.03), while caterpillars preferred old leaves (binomial probability for 9 of 10 = 0.01) but showed no preference for light or shade leaves (binomial probability for 6 of 10 = 0.2).
Leaf Comparison
Shade leaves were significantly larger than the light leaves (Figure 1 top; 2-way ANOVA, F = 44.02, df = 1,115, P < .0001). The stinging hair density and total number of the hairs for the light leaves were significantly higher than for the shade leaves (Figure 1 middle and bottom; hair density: 2-way ANOVA, F = 194.58, df = 1,115, P < .0001; total hairs: F = 94.67, df = 1,115, P < .0001). The hair density and leaf area did not explain each other, because the shade plants increased little in total hair counts with increasing age and leaf area (Figure 1 top). The slopes of increase in the hair density between the two treatments differed significantly (2-way ANOVA, F = 11.78, df = 1,115, P < .0001).
Discussion
The significantly lower leaf area and stinging hair count and density in the shade plants agreed with the predictions and therefore supported the hypothesis that U. dioica responds to the light availability by altering its physical and chemical defense system. The differences were significant between the two treatments in all age groups, suggesting that the response may begin even before putting forth the first leaf. These results run counter to the generalization by May et al. (6) that medicinal plants including Urticaceae lack adaptive phenotypic plasticity in regulating defense investments on the basis of light availability.
Interestingly, the number of stinging hairs increased little for the shade plants with age. One possibility is that the shade plants stalled production of hairs while the light leaves continued production, likely in response to the light availability (Figure 1 top). On the other hand, Agrawal and Spiller (7) found that tropic silver buttonwood recovering from a disturbance allocated fewer resources towards defensive trichomes and more towards leaf growth. U. dioica could be similarly investing fewer resources in hairs for the earlier leaves and more for the later younger leaves when the earlier leaves can provide photosynthates to support leaf production. Further studies would be needed to test for this mechanism.
The herbivory test revealed little or no preference for light leaves. This ran counter to the predicted preference for shade plants with limited resource for defense investment. One possible explanation is that the stinging hairs and the associated secondary compound in the hairs are targeted to another kind of herbivore-perhaps mammals. In this case, the light plants may have produced more hairs not only because of more resource availability but also because of greater exposure to mammalian herbivores. It may also be that there are other secondary metabolites in U. dioica that have different patterns with respect to sun and shade.
The preference of my test herbivores for old leaves shows that the physical structure of hairs is still effective and excludes them from young leaves with high density of hairs in both treatments. The herbivores did not exclude the stinging hairs from their consumption, however, and showed no sign of malignant consequences up to 36 hours after the trial, further suggesting that they may not be the intended target of the secondary metabolite in the hair.
The full complexity of U. dioica defense system remains unknown. My field observations indicate that the shade plants suffer from colonies of parasitic insects that did not seem to infest the light plants as much. Further studies would be required to test for the presence of other secondary metabolites and their anti-herbivore activity.
Acknowledgements
Research was done under the mentorship of Matt Ayres and Laurel Symes as a part of the Biology Foreign Study Program.
References
1. N. A. Lopatkin, A.V. Sivkov, A. A. Medvedev, Urologiia 12, 14-19 (2006).
2. M. R. Safarinejad, J. of Herbal Pharmacotherapy 5, 1-11 (2005).
3. M. El Haouari et al., Phytotherapy Res. 20, 568-72 (2006).
4. A. H. Gentry, A Field Guide to the Families and Genera of Woody Plants of Northwest South America (Conservation International, Washington, DC, 1993).
5. Uva, R. H., J. C. Neal, and J. M. DiTomaso, Weeds of the Northeast (Cornell University Press, Ithaca, NY, 1997).
6. May, C. H., S. L. Emel, and K. M. Sullan, Dartmouth Studies in Tropical Ecology, 54-57 (2006).
7. Agrawal, A. A. and D. A. Spiller, American J. of Botany 91, 1990-1997 (2004).