Sicong (George) Shan ‘23, Biology, 7/28/20
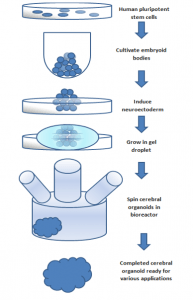
Figure 1: the flow chart shows the process of producing a cerebral organoid from pluripotent stem cells.
Source: Wikimedia Commons
Researchers have long been intrigued by the location restriction of inflammatory diseases in the human gastrointestinal (GI) tract. While Crohn’s disease (a kind of bowel disease that causes chronic inflammation of the GI tract) affects both the small intestine and the colon, it seems counterintuitive that ulcerative colitis (another bowel disease that causes chronic inflammation and ulcers) only appears in the colon since pathogens should be able to move freely through the GI tract and stimulate an immune response in many locations. Researchers at the University of Würzburg recently offered some insights into this puzzle as they discovered many of the GI tract immune signaling process in the epithelial layer to be site-specific and independent of the microbiota environment1. They achieved these results with the assistance of stem cell generated “mini-organ”2.
Until recently, researchers in tissue and organ biology were hindered by the accessibility of experimental subjects and, particularly with regard to human subjects, ethical concerns. However, developments in stem cell technology have now enabled in vitro study of animal and human tissue organoids. To create these organoids, a chosen type of stem cell is cultured and replicated on a special scaffold laced with molecular growth factors. This shapes the physical development of the organoid, which proliferates through self-organization and self-patterning3. Organoids are much smaller than their in vivo counterparts, with a typical diameter of only around 0.5 millimeters; however, they resemble most of the original 3D structure and functionalities1.
In the study by the University of Würzburg, organoids were generated from stomach, small intestine and colon cells to allow for comparison of cellular gene expression in each segment2. Differences in cellular gene expression served as evidence for site-specific immune signaling in the GI tract. Because derived organoids are devoid of independent immune cells, providing an undisturbed condition to monitor the production of immune signaling molecules by the gut epithelial cells alone.
Scientists performed experiments on 42 lines of human and murine GI organoids, from both adult and fetal tissues and from every segment of the GI tract1,2. RNA sequencing was conducted on each organoid to generate gene expression data while functional assays were used to analyze the expression data. In each species, gene expression differentiation existed between segments of the GI tract. Aside from typical genes marking the GI border, immune-related genes like Tlr1, Tlr2, Tlr4 were also found to have different expression levels. To verify whether the sequencing data translated into patterns in pathway activity, researchers conducted functional test on the Tlr4 pathway, a kind of Toll-like receptor that recognizes lipopolysaccharide. Expression of the inflammatory markers IL-8 in humans and Cxcl2 in mice were assayed to indicate activation of immune pathway like Tlr4, and the results aligned with the original sequencing data.
Additionally, experiments were conducted on murine organoids derived from embryonic stem cells to test the hypothesis of innate development of epithelial immune pathway. Only mice embryos were utilized due to the ethical concerns to using human embryos. Sequencing data and functional assays revealed a similar differentiation pattern of immune-related gene expression in the fetal GI epithelial cells. This finding on fetal GI gene expression plays down the effect of the postnatal exposure of microbiota on site-specific epithelial immune response in the GI tract, although it does not rule out the possibility. That being said, just because this observation was made in mice does not mean it applies to humans. Nonetheless, scientists can now conduct further studies, based on the findings of this research, into the molecular basis of regional activities of the GI epithelial immunity. This will hopefully shed light onto more effective therapies for inflammatory bowel diseases2.
Reference
1.University of Würzburg. (2020, July 6). Location, location, location: Even gut immune response is site-specific. ScienceDaily. Retrieved July 16, 2020 from www.sciencedaily.com/releases/2020/07/200706100820.htm
2.Kayisoglu, O., Weiss, F., Niklas, C., Pierotti, I., Pompaiah, M., Wallaschek, N., Germer, C.-T., Wiegering, A., & Bartfeld, S. (2020). Location-specific cell identity rather than exposure to GI microbiota defines many innate immune signalling cascades in the gut epithelium. Gut, gutjnl-2019-319919. https://doi.org/10.1136/gutjnl-2019-319919
3.Rossi, G., Manfrin, A., & Lutolf, M. P. (2018). Progress and potential in organoid research. Nature Reviews Genetics, 19(11), 671–687. https://doi.org/10.1038/s41576-018-0051-9